Acetic acid ordinarily would be in a solution. Acetic acid is a weak acid, but it can be very concentrated. The acetic acid component interacts favorably with water, while the methyl group does not, thus weakening the intermolecular forces.Intermolecular forces (IMF) (or secondary forces) are the forces which mediate interaction between molecules, including forces of attraction or repulsion which act between atoms and other types of neighboring particles, e.g. atoms or ions.Intramolecular and intermolecular forces. This is the currently selected item. Intramolecular and intermolecular forces. Google Classroom. Facebook.The role-effect of the intermolecular forces (intermolecular bonding) involved and the their effect on the boiling point is explained and Good idea to first read 8.2.1 A summary of Van der Waals forces, an introduction to intermolecular forces.Intermolecular forces hold multiple molecules together and determine many of a substance's properties. Under certain conditions, molecules of acetic acid, CH3COOH, form "dimers," pairs of acetic acid molecules held together by strong...
Intermolecular force - Wikipedia
Intermolecular forces (forces between chemical species) are important in biochemistry. Many functional groups have distinctive odors. Small carboxylic acids smell like acetic acid (vinegar), while larger ones have unpleasant odors.Intermolecular forces - liquids and intermolecular forces The strengths of intermolecular forces in different substances vary over a wide Hence, the boiling point of acetic acid is higher. These effects can be important...Between two molecules of acetic acid there are 3 forces of attraction that affect them. These being the London dispersion force, the dipole-dipole force and hydrogen bonding. The first force of attraction is the london dispersion force.The intermolecular forces when I mix acetic and B occur in one phase and therefore acetic acid which is also polar Choice A: Acetic acid molecules alone, nothing else, pure: dipole-dipole intermolecular forces Choice B: Acetic acid...

Intramolecular and intermolecular forces... | Khan Academy
Intermolecular forces, Van der Waal's forces, hydrogen bonds, dipole-dipole interactions and dispersion or London forces, and how these effect the physical properties of covalent substances tutorial for chemistry students.Learn what intermolecular forces are, understand the 3 types of intermolecular forces, and get examples of each type. 3 Types of Intermolecular Forces. Forces That Determine How Molecules Behave....Forces: Acetic Acid HCN C2H6 (CH3COOH) Hydrogen bonding Dipole-Dipole Dispersion forces Dipole-dipole Dispersion Summary Intermolecular Forces Polarity Types of Intermolecular Forces Strength Nonpolar Dispersion Weak...Intermolecular forces. Why does water bead up like this on certain surfaces, like a waxed car or glass? Intermolecular forces are the glue that hold many materials together. They give many substances their properties, such as melting and boiling...The forces between molecules that bind them together are known as Intermolecular forces allow us to determine which substances are likely to dissolve in other Propanoic acid has hydrogen bonds which are much stronger than the...
Acetic acid = ethanoic acid, which has the components CH3COOH. ('eth' = 2 carbons, and 'oic' method it is has the carboxylic functional group, which is COOH).
The presence of Hydrogen and a member of the NOF parts (Nitrogen, Oxygen, Fluorine) - in this case oxygen - method it has hydrogen bonding.
Hope that is helping :)
b Identify the types of intermolecular forces that exist ...

6. Properties of carboxylic acids - Alcohol, carboxylic ...

Chemistry Archive | October 27, 2016 | Chegg.com

Hydrogen Bonds - INTERMOLECULAR FORCES

TASK 1 Experiment: Preparation of esters Teacher`s Memorandum

Solved: Identify The Dominant Intermolecular Attraction In ...

What is the dominant intermolecular force in ethanoic acid ...
Chemistry Works: Hydrogen Bond

Under certain conditions, molecules of ace... | Clutch Prep

Hydrogen Bonding - INTERMOLECULAR FORCES

Ethyl ethanoate ethyl acetate Methyl propanoate 4 ...

Intermolecular Forces - Chemistry LibreTexts

Hydrogen Bonding - INTERMOLECULAR FORCES

Hydrogen Bonding, Chemistry Study Material @eMedicalprep ...

Hydrated protons pair off - Wikinews, the free news source

chemistry -organic molecules-trends in boiling temp ...

Solved: 3 5 6 7 8 10 Decide Which Intermolecular Forces Ac ...

Intermolecular Forces Review | Organic Chemistry Help

SOLVED:What type of intermolecular forces must be…

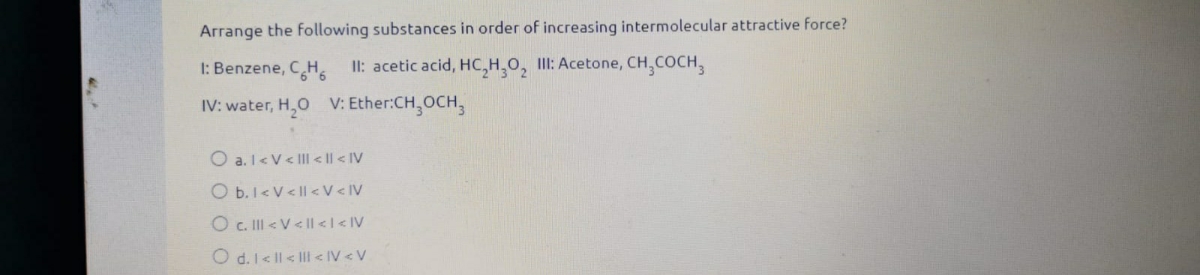
Answered: Arrange the following substances in… | bartleby
0 Comment to "What Is The Dominant Intermolecular Force In Acetic Acid?"
Post a Comment