At the half-equivalence point, the solution is a buffer with equal amounts of weak acid and its conjugate weak base. After the equivalence point, the stoichiometric reaction has neutralized all the sample, and the pH depends on how much excess titrant has been added.Question: What Is The PH At The Half-equivalence Point In The Titration Of A Weak Base With A Strong Acid? What is the pH at the half-equivalence point in the titration of a weak base with a strong acid?At half equivalence point, the concentrations of the weak acid and its conjugate base are equal. So that ratio is 1. The log of 1 is zero, so, the pH = pKa.Half equivalence point. We can just sketch approximate titration curves of pH vs. percent of titration, since we do not have the We can, however, precisely determine the pH at the half-equivalence point [mid-way between untitrated and completely titrated for a weak acid (or base)] this is equal to...Attempts to measure that pH at the equivalence point are doomed to failure because at this point the pH will be very sensitive to tiny additions of base This means that the concentration of the conjugate base is only half what it was to begin with: 0.005M. It is as if the original acid had been diluted 2:1...
Solved: What Is The PH At The Half-equivalence Point... | Chegg.com
How do I find Ka from pH at equivalence point? In my chem lab, I titrated an unknown acid with NaOH; the pH at equivalence point is around 7.8 and this occurs at 24.4 mL of NaOH added. The concentration of NaOH is 0.10002 M. the total amount of acid titrated is 25 mL.The half-equivalence point of a titration occurs half way to the end point, where half of the analyte has reacted to form its conjugate, and the other half stillremains unreacted. If 0.200 moles of a monoprotic weak acid (Ka = 1.8x10-5) is titrated with NaOH, what is the pH of the solution at the half...And so at the half equivalence point, the solution will contain equal numbers of moles of the weak acid and of its conjugate base, which implies that you're now dealing with a buffer solution. As you know, the #"pH"# of a weak acid-conjugate base buffer can be calcualted using the Henderson...The starting pH is 13.3. Add your answer and earn points.

At half equivalence point, why does pH=pKa? | Yahoo Answers
My book says that you cannot use the Henderson-Hasselbalch equation to find the pH at the equivalence point when titrating a weak acid with a strong...I learned in class that the equivalence point in an acid-base titration is reached when the solution contains an equal amount of substance of $\ce The equivalence point is reached when all ammonia has been converted to ammonium ions. The result, at the equivalence point, will be the same as...Titration Why Is Double My Half Equivalence Point Not Equal To My Equivalence Point.Note: pH = pKa at the half-equivalence point. Remember that. You stand a very good chance of being asked a half-equivalence point question on your test. Solution to part (e): The solution is now completely composed of a salt of a weak acid.At HALF-WAY equivalence point the pH = pKa, since the actual concentration of ACID is equal to concentration of its conjugate BASE, because both are equal to HALF of the original (= unknown analyte) concentration to be titrated (half left = half formed).
You can glance at both the Henderson-Haselbalch equation or at the expression for Ka. For the H-H equation,
pH = pKa + log [base]/[acid]
where "base" and "acid" are the weak acid and its conjugate base.
At half equivalence point, the concentrations of the vulnerable acid and its conjugate base are equivalent. So that ratio is 1. The log of one is 0, so, the pH = pKa.
Hope this is helping...
Titration HNO3 with NaOH | scatter chart made by Zhaoay ...

half of the weak acid has been converted to its conjugate ...

chads chapter 5 - AP Chemistry None with None at Syracuse ...

Solved: The Half-equivalence Point Of A Titration Occurs H ...

How can you experimentally determine the pK_a of acetic ...

TITRATION - Equilibrium, Acids and Bases, Titrations, and ...

Sweetsugarcandies: Weak Acid Strong Base Titration Curve ...
Solved: First And Second Derivative Plots For Titration Cu ...

Why does the pH before the equivalence point of a ...

Acids & Bases Flashcards | Quizlet

14.7: Acid-Base Titrations - Chemistry LibreTexts

Chemistry Chapter 7 {Acids and Bases} at University of ...

Chem 101:Mod C- Titrations Lect#7 at Curtin University of ...

Titration Curve weak base with strong acid START

Solved: Show PH Vs Vol Of NaOH In ML Graph For Trial 2 In ...

Solved: 1) The Half-equivalence Point Of A Titration Occur ...

Solved: Data Table 1: Equivalence Point Trial 1 Trial 2 Vo ...

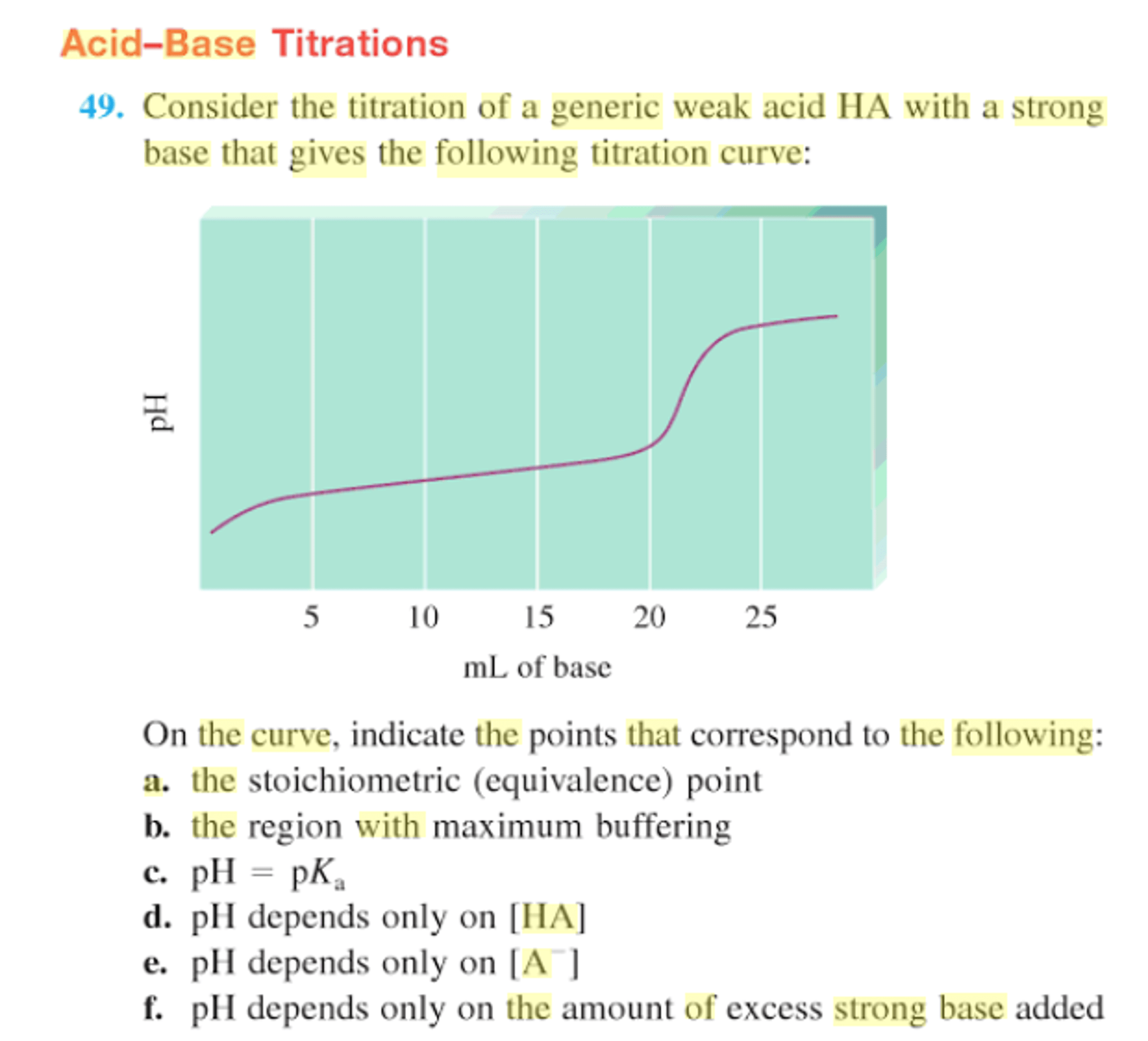
Solved: 10. Label On Your Titration Curve Each Of The Foll ...

CHM 2046C Module 12 Part D: Acid-Base Titration Curves

Acid-base titration

Why Does Ph Equal Pka At Half Equivalence Point

0 Comment to "Titration Of A Weak Acid With A Strong Base - Chemistry LibreTexts"
Post a Comment